Without catalysts, humans wouldn’t have got far. They trigger many of the processes on which we rely, from age-old mechanisms deep down in our cells to the production of contemporary consumer goods. They could also smooth our path towards a sustainable future. EU-funded researcher Xile Hu has delivered promising advances.
New catalysts could help to unlock the potential of many innovations, notably by making key processes less costly and reducing the waste they generate. Xile Hu of Switzerland’s Ecole polytechnique fédérale de Lausanne has taken the technology another step ahead.
His research focused on two main challenges: the development of affordable and effective catalysts to produce hydrogen from water using a solar-powered process, and the replacement of the costly catalysts used to synthesise various high-value chemicals by more affordable alternatives that also produce less waste.
Hu benefited from an ERC Starting Grant for CAT4ENSUS. The European Research Council (ERC) provides this type of funding to support promising scientists with excellent research proposals who intend to set up research teams in an EU member state or an associated country. The proposal submitted by Hu, a Chinese scientist conducting research in Switzerland, ticked all the boxes.
Activating sustainable reactions
“Catalysts are used for many reactions that would otherwise be very slow, or would not happen at all,” says Hu. “In the presence of suitable catalysts, these processes unfold very quickly.”
And indeed, different processes require different catalysts, many of which are based on expensive and rare metals such as ruthenium, palladium or platinum. Finding more common and affordable substances to set these processes in motion would help to make them less costly and run them sustainably on a much larger scale.
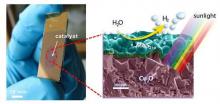
This possibility could contribute to eagerly awaited technological breakthroughs — such as the development of an inexpensive, environmentally friendly process for the production of hydrogen. As a clean and potentially abundant energy carrier, this substance could help to reduce our reliance on fossil fuels, but in fact most of the hydrogen produced at the moment is derived from natural gas.
Hu’s team was determined to make its contribution to the advent of the hydrogen economy. Instead of the platinum catalysts that predominate in hydrogen production, it uses molybdenum, combined with sulphur. “Molybdenum is about a thousand times less expensive than platinum, and a thousand times more abundant,” says Hu.
This catalyst is intended for a solar-powered process that extracts hydrogen from water. While many other technologies have to come together to transform this approach into an exploitable technology, says Hu, CAT4ENSUS has already achieved very promising results at the lab scale. It is thus paving the way towards affordable and sustainable production, conversion, storage and transport of solar energy.
Creative chemistry
Developing new catalysts requires both information and inspiration, says Hu. "The relevant chemical reactions have to be analysed rigorously," he explains, "and once they are fully understood, ideas on innovative processes and on ways to produce interesting molecules begin to emerge."
The CAT4ENSUS team used this approach to develop iron-catalysed processes for the synthesis of high-value molecules, notably for the pharmaceutical industry and for use in perfumes. “Iron is great for this purpose. It is not toxic, and it is one of the most abundant elements on earth and therefore really cheap,” Hu explains.
The new catalysts are more affordable than the ones used to date, he reports, and the processes they support are designed to generate as little waste as possible. Several of these new catalysts and processes are already used by the private sector.
CAT4ENSUS is due to end in December 2015, and Hu is planning the next steps. The catalyst he and his team designed for the production of green hydrogen will need to be integrated with sufficiently innovative components to support the process, as soon as they become available. This will require extensive research and development, as he notes. And, of course, there are many more processes to create or optimise for the production of exciting, innovative molecules.